BREAKING THE 80% RULE
Rules were meant to be broken – Giving batteries life beyond 80%
The 80% Rule
We know that batteries do not last forever. If you own a mobile phone, you’ve probably experienced this issue around the 2-year mark. Your device is fully charged when you wake up, just for it to die halfway through the day. You wonder, “How is this possible?”
Well, your battery probably reached its end of life – which happens to be after it has used only 20% of its total lifetime energy.
Conventional Li-ion battery wisdom, and manufacturer warranties, deem a battery is “dead” after capacity fade brings the battery to 80% of its original capacity. Capacity fade is the gradual loss of a battery’s output energy over time.
At 80% nominal capacity, 2 things can happen: the rate of capacity loss makes the usable capacity too little for future cycles, or the rate of capacity loss accelerates making the battery drain much faster and/or become unpredictable.
This is commonly known as the 80% rule.
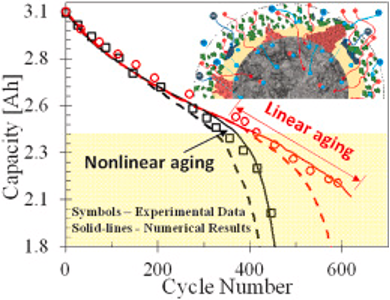
Figure 1. Illustration showing the two aging regimes batteries undergo after reaching 80% capacity1.
Why do batteries lose capacity?
Batteries are not designed to fail at this point in their life. Several degradation mechanisms contribute to capacity loss, and positive feedback loops between different mechanisms can snowball once the battery reaches the 80% capacity threshold, making performance beyond 80% difficult to predict. Figure 1 shows that batteries can gradually lose their capacity after this point (linear aging), or very rapidly lose it (nonlinear aging). This rapid loss is evidenced by the so-called “knee-point.”
Many factors contribute to capacity loss in cells (Figure 2). Nearly every component has some type of degradation mechanism that might cause the battery to perform worse over time. While it is difficult to control every type of failure method, we can attempt to mitigate the main contributors. Two loss factors that we have strategies for protecting against are active material dissolution and loss of lithium from the electrolyte.
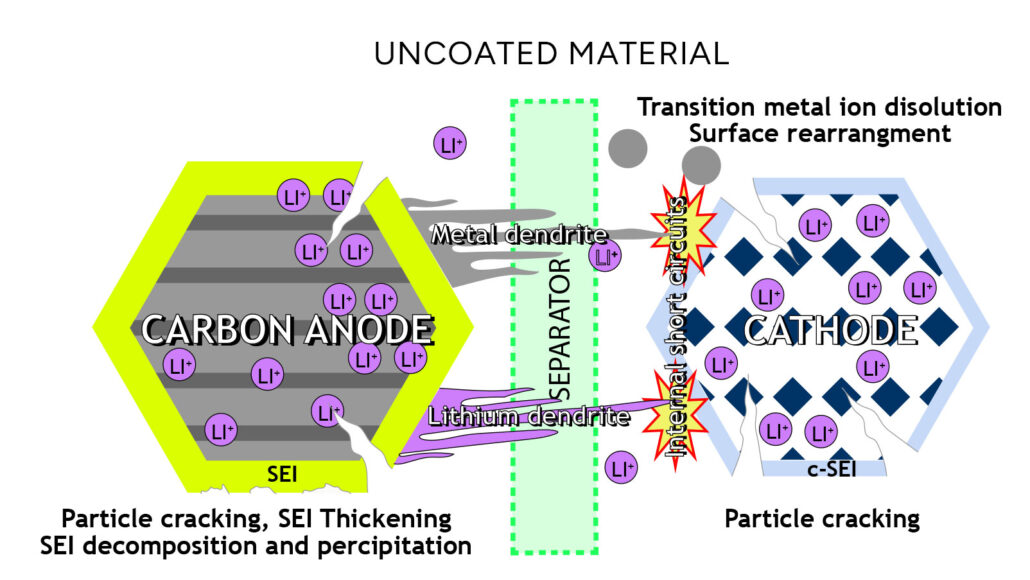
Figure 2. Overview of degradation mechanisms in a lithium-ion battery.
How can we protect batteries from these degradation methods and delay rapid capacity loss as long as possible?
At Forge Nano, we are breaking the 80% rule by protecting electrode materials from fast capacity loss with a solution called Atomic Armor™.
Atomic Armor is essentially a very thin shell, less than 5 nm thick, that surrounds individual electrode particles. It is applied with a method called atomic layer deposition, or ALD, which results in extremely precise thickness growth and a highly robust coating.
As a barrier, Atomic Armor protects the active material against parasitic reactions with the electrolyte, which can be chemically unstable when the battery operates at increased voltages and temperatures. It is under these conditions, such as those a battery is subject to when fast charging, that can cause rapid capacity loss.
Figure 3 illustrates a battery that has had its cathode and anode coated with Forge Nano’s Atomic Armor. The nanometer-thick shell acts as an artificial solid electrolyte interface (SEI) by preventing the loss of lithium ions that would typically be used to form the SEI. It also stabilizes the electrode structure to keep the particle from cracking and stop other metals, like Mn2+, from migrating from the crystal structure.
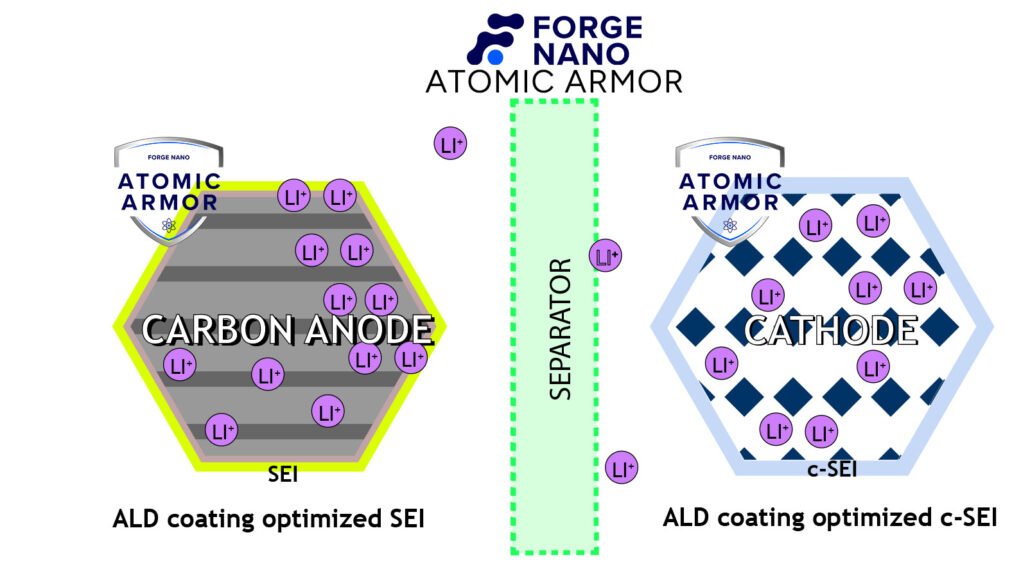
Figure 3. Ideal operation of a Li-ion battery with coated electrodes preventing failure mechanisms as seen in Figure 2.
What does Atomic Armor have to do with the 80% rule?
Let’s look at how batteries typically age without protective coatings and an example of a battery aging that has been equipped with Atomic Armor.
Figure 4a shows the normal operation of 48 NMC811/graphite cylindrical cells with durability cycling of 1C/1C at 25 °C. Across all voltages, the knee point began between 80-85% of capacity retention. For all 48 cells, the capacity loss to the batteries’ end-of-life proceeded rapidly after the knee point. This loss behavior is quite common, especially in more unstable conditions, and corroborates the 80% rule warranties of battery manufacturers.
Conversely, Figure 4b shows the cycling behavior of a cell where both the anode and cathode have been coated with Forge Nano’s Atomic Armor. The addition of Atomic Armor drastically improved the battery’s loss profile, cycling consistently well past 80% of the original capacity. Even after 80%, the loss proceeded at a much more predictable, linear rate with no signs of a knee point.
Further supporting the use of Atomic Armor, the durability cycling of the cell was done at 4C/1C, representing fast charge conditions, i.e., a more extreme environment than what the cells in Figure 4a were subject to. Even at a higher C rate, Atomic Armor allows the battery to age slowly and predictably.
Through a combination of a stable SEI, stabilization of the electrode structure and robust barrier properties, Atomic Armor prevents the rapid consumption of active material and electrolyte that typically causes snowballing failure of batteries.
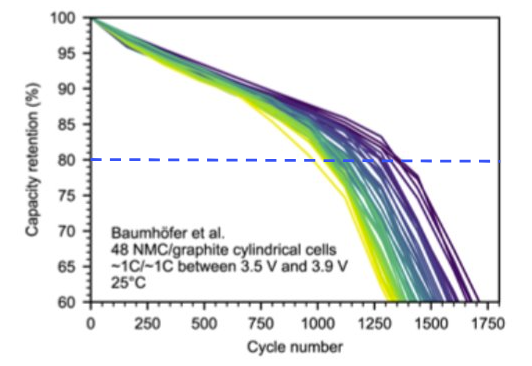
Figure a
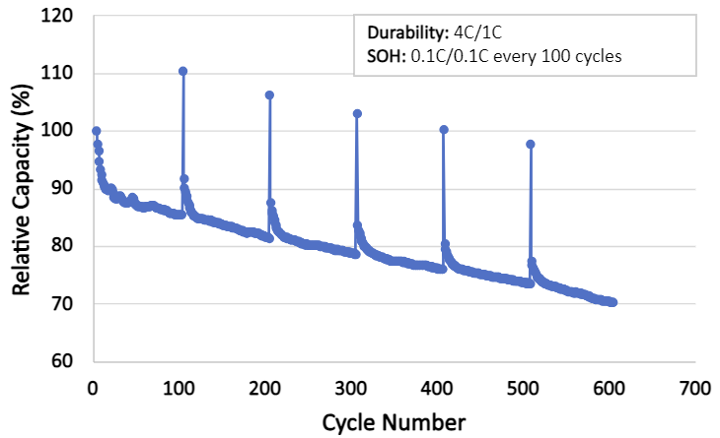
Figure b
Figure 4. (a) Capacity retention vs cycle number for 48 commercial NMC/graphite cylindrical cells. Adapted from Attia et al. Figure 172. (b) Capacity retention of a cell containing electrodes coated with Atomic Armor in fast charge conditions.
We must only accept limitations until we find a way to break them.
This markedly slower degradation of the cell capacity dispels the notion that we must replace a battery after it reaches 80% nominal capacity. With Forge Nano’s Atomic Armor, Li-ion batteries will last longer and be used closer to their full potential than ever before.
Not only can we use our batteries longer, but we also enable their use in more extreme conditions, like fast charging, as well as provide a solid business case for second-life batteries (when batteries from EVs get removed and then used for grid-storage).
ALD will be a necessity for future battery manufacturing. Limited availability of lithium supplies, increasing demand for batteries, and an obligation to limit waste make it our responsibility to push the potential of our materials as much as possible. The extended lifetime of lithium-ion batteries will have far-reaching implications for controlling a reliable Li supply chain and staving off the worst effects of rapid growth in lithium demand.
Join us as we work to tear down as many assumptions about battery performance as possible.
